
A significant quality issue was identified with the YH82 FG components — cosmetic residual glue and surface scratches. The issue compromised the aesthetic integrity of the product, resulting in a 22.5% fallout rate, which prompted an urgent response from both the client and the manufacturer.
During a routine inspection by the client's Incoming Quality Control (IQC) team, a batch of YH82 FG components was identified as having quality issues. Two primary defects were reported:
Visible residual glue on the product surface
Multiple scratches affecting the cosmetic integrity of the product
These flaws were particularly concerning for a product where visual aesthetics matter to end customers. The issue meant the components were out of compliance with cosmetic standards, resulting in a large-scale rejection and necessitating immediate containment and rework.
Upon learning about the issue, the manufacturer swiftly implemented a set of containment measures to stop the problem from escalating further.
A total of 6,400 pieces that had been delivered to the client were immediately rejected and isolated. Rework personnel were sent to the client site to handle the defect remediation directly. The fast response helped ensure that none of the defective components made it into the client’s production line.
Within the manufacturer’s inventory, 320 PCS of the same product were identified. These units were quickly sealed with red labels and inspected. The findings were:
10 PCS exhibited residual glue
1 PCS showed signs of collision damage
The inventory was quarantined, and necessary rework procedures were initiated to bring the parts up to standard.
A critical lesson emerged during this phase: a communication failure had occurred. The 6,400 in-transit units had not been reported or flagged to the client in time, leading to client-side complaints and damage to trust. The issue was recognized as an internal breakdown in quality communication and was escalated for a process review.
To manage the issue more effectively and ensure cross-functional collaboration, the company established a Quality Improvement Team (QIT).
The QIT was responsible for:
Driving the root cause investigation
Coordinating between departments (production, logistics, QA, and engineering)
Developing and enforcing corrective and preventive actions
Monitoring the long-term effectiveness of implemented improvements
The team included representatives from:
Quality Assurance
Production Operations
Logistics and Supply Chain
Engineering
Client Relations
Our Team Member includes the following name, they have professional experience and know best troubleshooting skills.
|
No. |
Name |
Function |
|
Role Description |
|
1 |
Eric Yang |
Leader |
Improvement team leader (responsible for abnormal progress work) |
|
|
2 |
Smart Cao |
Manager |
Team member (abnormal project initiation and summary) |
|
|
3 |
Peng Li |
OQC |
Team member (abnormal improvement project follow-up) |
|
|
4 |
Lotus Zhang |
IQC |
Team member (implementation and execution of abnormal improvements) |
|
|
5 |
Alice Shi |
CS |
Team member (internal and external communication and coordination) |
|
|
6 |
Evan Qi |
Sales |
Team member (internal and external communication and coordination) |
|
|
7 |
Lily Zhang |
Director |
Team member (internal and external communication and coordination) |
|
|
8 |
Deric Wang |
Engineer |
Team member (technical support for abnormal improvement) |
|
|
9 |
Jason Yang |
CTP |
Team member (technical support for abnormal improvement) |
This team structure ensured all aspects of the problem—technical, operational, and client-facing—were addressed holistically and with accountability.
Despite the defects being severe, the team was fortunate that the problem was caught so early.
The defective components were identified before being integrated into the client’s production processes.
All defective materials were isolated and subjected to timely rework.
No defective products reached end customers or entered the market.
As a result, there was no direct impact on customers or consumers, and the risk of broader brand damage was avoided.
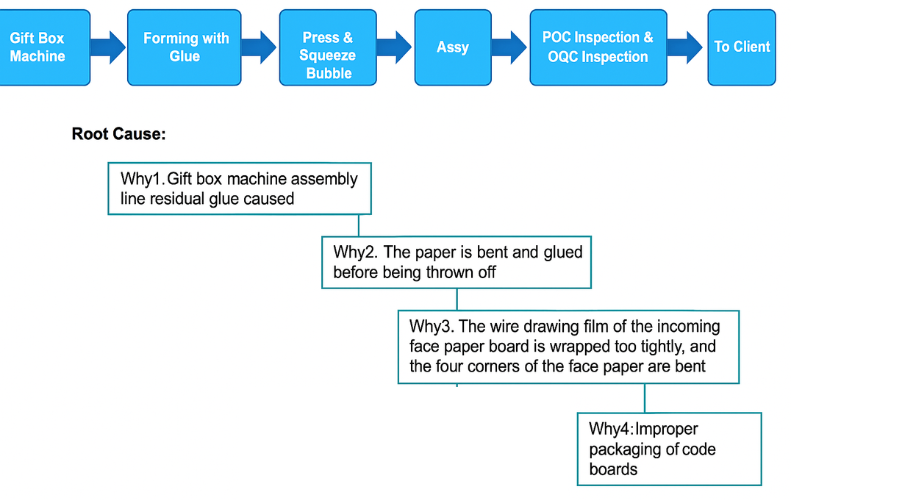
To prevent recurrence, the team conducted a detailed investigation, focusing on the upstream processes that might have contributed to the issues.
The primary issue was traced back to the Tianhe paper operation, a critical step in the component's surface treatment. The findings included:
The drawing film (code plate film) was applied too tightly, especially at the corners.
This tension caused the paper corners to bend slightly.
Bent paper led to uneven glue application, resulting in residual glue on the surface.
During transport and subsequent production stages, rubbing occurred, compounding the cosmetic defects.
Beyond the deformation during production, further problems were found in how components were handled:
The packaging of incoming paper pallets was not standardized, causing physical damage even before processing.
The stacking method for semi-finished products lacked protective measures, making them vulnerable to scratches and impact damage during handling and storage.

With the causes identified, a targeted improvement plan was developed. The goal was not only to address the immediate issue but also to implement systemic changes within the manufacturing and quality assurance processes.
Loose controls on how glue was applied and cleaned during production.
Improper packaging and handling of materials at several stages.
Inadequate inspection routines during in-line processing failed to catch problems early.
Logistics personnel underwent focused training on handling and packaging specifications. Key changes included:
Ensuring that drawing films are applied with appropriate tension, especially around corners.
Addressing any observed bending in face paper before use in production, by reversing the paper direction or pre-treating it.
The PQC (Process Quality Control) team now performs pre-production checks on all incoming face paper. The production line only accepts materials that pass a check for curling, bending, or deformation.
A “2-hour wiping rule” was enforced, requiring workers to clean the production line at regular intervals.
Quality personnel conduct random inspections along the line to detect and address any residual glue issues proactively.
Pad paper is now used to provide interlayer protection for all semi-finished product stacks.
All such products must undergo final appearance inspection for scratches or collision marks before approval for the next stage or shipping.
The containment and corrective actions produced immediate positive results:
All affected products were successfully reworked and returned to the client.
The quality team has standardized new procedures across departments, ensuring consistency.
A comprehensive material-level review is now part of the final quality approval process, enabling proactive detection of anomalies.
|
No. |
Action Description |
Result |
Check Date |
DRI |
Status |
|
1 |
Follow up on the quality of the finished product packaging status |
OK |
2025-03-23 |
Eric |
OK |
|
2 |
Ensure no curling or bending of the facial tissue due to excessive moisture |
OK |
2025-03-23 |
Eric |
OK |
|
3 |
Update and issue the relevant SOP/SIP; ensure site personnel implement improvement measures on time |
OK |
2025-03-23 |
Eric |
OK |
|
4 |
Assess and handle responsible individuals; provide educational and warning impact as a corrective measure |
OK |
2025-03-23 |
Eric |
OK |
This structured response allowed the company to regain the client’s trust while also strengthening its internal quality systems.
This case study highlights the crucial importance of responding promptly and effectively to a quality crisis. Even issues as seemingly superficial as cosmetic glue and scratches can become significant problems when they disrupt production or damage client relationships.
By forming a Quality Improvement Team (QIT) and following a clear framework — including containment, root cause analysis, corrective action, and verification — the team turned a potential escalation into an opportunity for improvement.
Moreover, the lessons learned extended far beyond the YH82 FG product line. New training programs, inspection standards, and communication protocols are now in place to ensure long-term quality and reduce risk across the board.